cross-posted from: https://lemmy.world/post/8344778
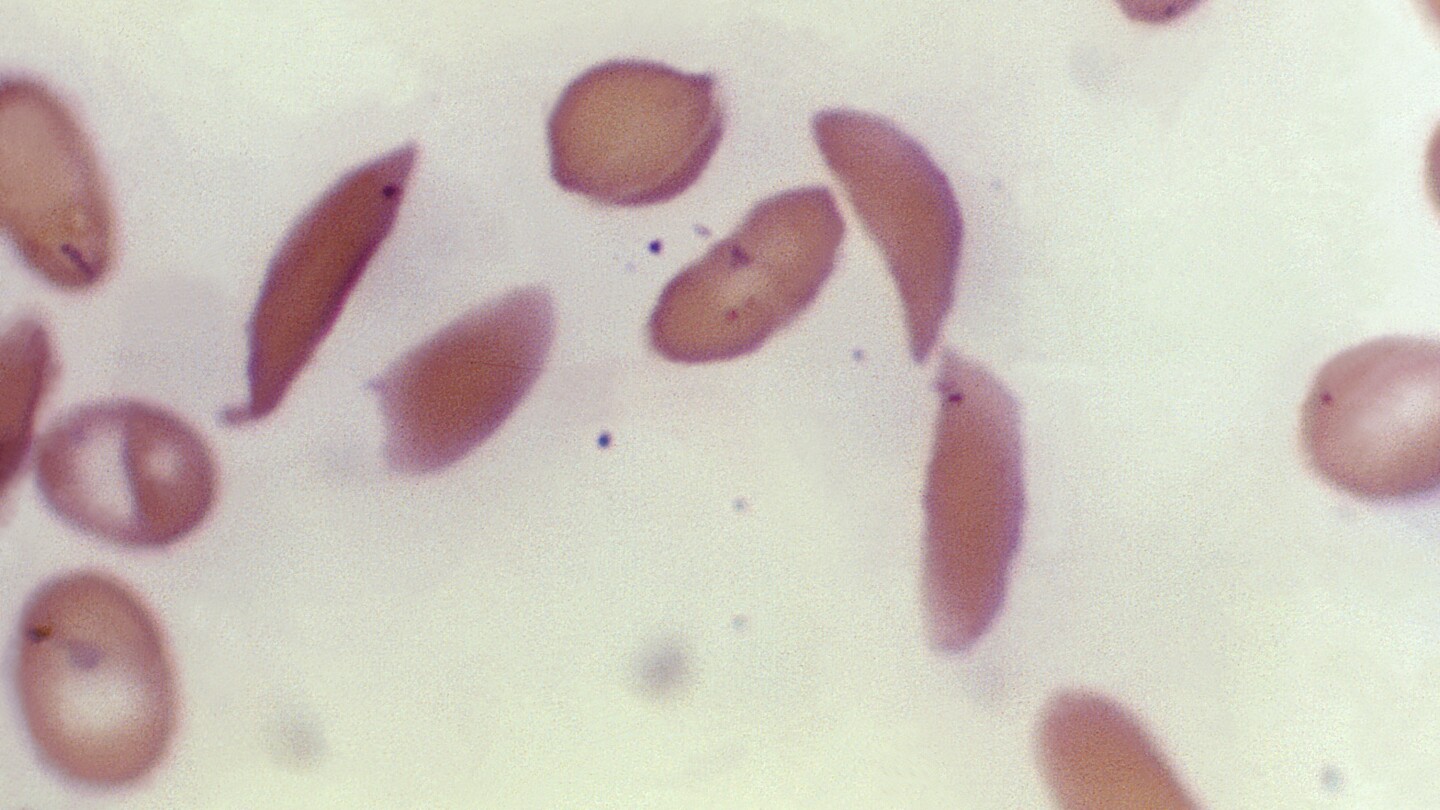
Britain’s medicines regulator has authorized the world’s first gene therapy treatment for sickle cell disease, in a move that could offer relief to thousands of people with the crippling disease in the U.K.
In a statement Thursday, the Medicines and Healthcare Regulatory Agency said it approved Casgevy, the first medicine licensed using the gene editing tool CRISPR, which won its makers a Nobel prize in 2020.
The agency approved the treatment for patients with sickle cell disease and thalassemia who are 12 years old and over. Casgevy is made by Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics. To date, bone marrow transplants, extremely arduous procedures that come with very unpleasant side effects, have been the only long-lasting treatment.
“The future of life-changing cures resides in CRISPR based (gene-editing) technology,” said Dr. Helen O’Neill of University College London.


This is the best summary I could come up with:
“The use of the word ‘cure’ in relation to sickle cell disease or thalassemia has, up until now, been incompatible,” she said in a statement, calling the MHRA’s approval of gene therapy “a positive moment in history.”
The new medicine, Casgevy, works by targeting the problematic gene in a patient’s bone marrow stem cells so that the body can make properly functioning hemoglobin.
Britain’s regulator said its decision to authorize the gene therapy for sickle cell disease was based on a study done on 29 patients, of whom 28 reported having no severe pain problems for at least one year after being treated.
Gene therapy treatments can cost millions of dollars and experts have previously raised concerns that they could remain out of reach for the people who would benefit most.
Vertex Pharmaceuticals said it had not yet established a price for the treatment in Britain and was working with health authorities “to secure reimbursement and access for eligible patients as quickly as possible.”
Casgevy is currently being reviewed by the U.S. Food and Drug Administration; the agency is expected to make a decision early next month, before considering another sickle cell gene therapy.
The original article contains 774 words, the summary contains 194 words. Saved 75%. I’m a bot and I’m open source!